Gel Filtration Chromatography
Principles of GFC
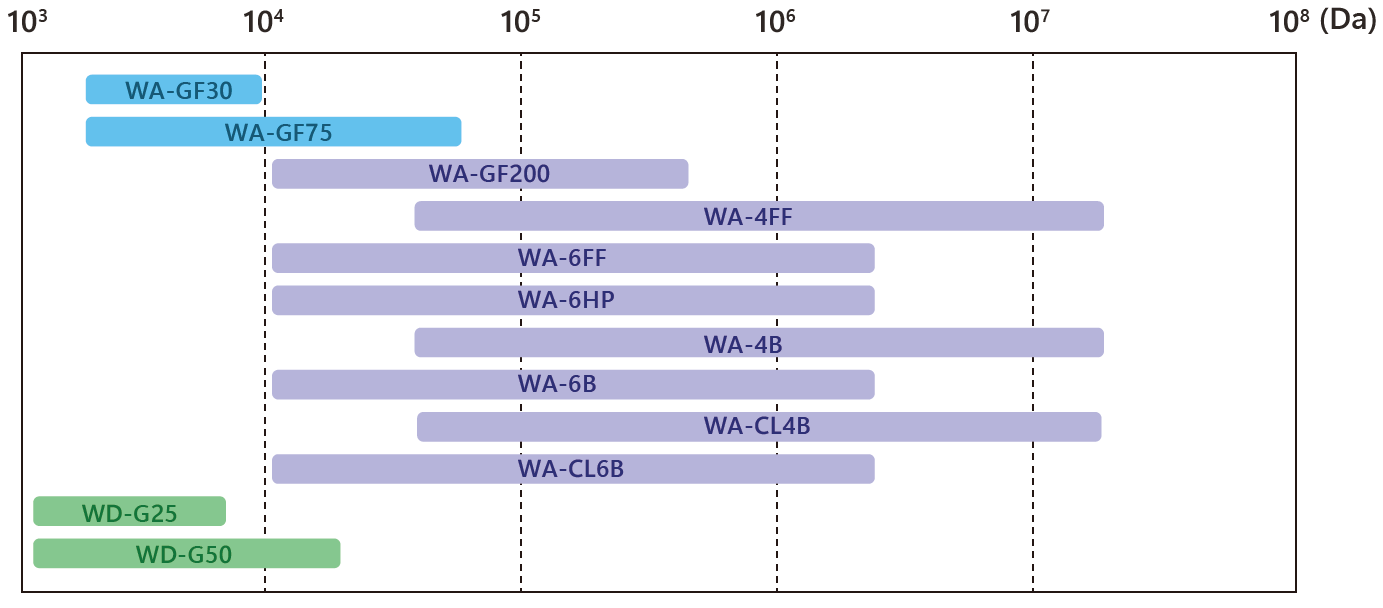
Gel filtration chromatography (GFC) separates biomolecules based on differences in size and shape. The media consists of porous beads. Large molecules cannot enter the internal pores and elute quickly with the void volume, resulting in a shorter retention time. Smaller molecules penetrate the pores and elute later, as they spend more time within the matrix. Effective separation depends on choosing media with suitable pore sizes according to the molecular weight of the target substances.
Choosing Gel Filtration Resin-Based on Separation Purpose
- Group Separation
This involves classifying sample components into high and low molecular weight groups. It is commonly used for desalting and buffer exchange. Example: WD-G25 series. - Fine Separation
This aims to separate components with similar molecular weights and is used in analytical or fine purification stages. Example: WA-FF and WA-GF series.
Separation Range of Gel Filtration Media (for Globular Proteins)
Ion Exchange Chromatography
Principles of IEC
Ion exchange chromatography (IEC) separates biomolecules based on differences in their isoelectric points. At a given pH, different biomolecules carry different types and amounts of charges. This technique utilizes these differences for separation. Depending on the type of functional groups, ion exchange chromatography is categorized into cation and anion exchange chromatography.
Key Features:
- High controllability
- Strong selectivity
- High recovery rate
- High loading capacity
- Sample concentration capability
Ligands for IEC
| Ligand | Group | Category |
|---|---|---|
| S | Sulfomethyl | Strong cation exchange |
| SP | Sulfopropyl | Strong cation exchange |
| CM | Carboxymethyl | Weak cation exchange |
| Q | Quaternary ammonium | Strong anion exchange |
| DEAE | Diethylaminoethyl | Weak anion exchange |
Hydrophobic Interaction Chromatography
Principles of HIC
Hydrophobic interaction chromatography (HIC) separates substances by leveraging interactions between hydrophobic groups on biomolecules and hydrophobic ligands on the stationary phase. Salt ions disrupt the hydration shell around biomolecules, promoting interaction with hydrophobic ligands. Common ligands include butyl thioether, butyl, octyl, and phenyl groups.
Advantages
- Sample loading under high salt, elution under low salt—well-compatible with ion exchange chromatography
- Strong selectivity
- Excellent physicochemical stability
Factors Influencing HIC Performance
1. Salt Type and Concentration
Salts increase interfacial tension between proteins and resins, disrupting hydration layers and enhancing hydrophobic interactions, thereby promoting protein binding to the matrix.
Salts exert varying effects on HIC. Sulfates like Na₂SO₄, K₂SO₄, and (NH₄)₂SO₄ enhance hydrophobicity by strengthening protein salting-out effects and surface tension, whereas MgCl₂, while also strengthening surface tension, increases solubility and hence diminishes hydrophobicity and hinders protein binding.
High salt concentrations enhance hydrophobic interactions. A salt concentration near but below the target protein’s salting-out point optimizes binding without causing precipitation. Typically, a 1 mol/L ammonium sulfate solution is sufficient to promote binding of highly soluble proteins to resins.
2. pH
HIC typically uses neutral phosphate buffers. While pH changes have limited effect, higher pH may slightly reduce protein hydrophobicity. pH adjustments are rarely used for modulating HIC.
3. Temperature
Higher temperatures enhance hydrophobic interactions but may compromise protein activity. HIC should be performed under stable temperature conditions to avoid unwanted influences.
Affinity Chromatography
Principles of Affinity Chromatography
Affinity chromatography exploits the specific and reversible interactions between biological molecules-such as antigen-antibody, enzyme-substrate, hormone-receptor, or complementary DNA strands-to achieve high-purity separations.
Advantages of Affinity Chromatography
- Fast and efficient
- High selectivity
- Achieves >90% purity in a single purification step
Resin Structures
- Matrix
A solid support for ligand immobilization, typically agarose or dextran gels - Spacer Arm
A linker of appropriate length connecting the matrix to the ligand - Ligand
A molecule capable of specifically and reversibly binding the target biomolecule
Empty Columns
WK-EC 6.6/10 Series
WK-EC 6.6 Series and 10 Series are lab-scale empty columns specifically designed for medium- and low-pressure chromatography. They are applicable to biomolecules such as recombinant proteins, antibodies, vaccines, and blood products, as well as small molecules like antibiotics, peptides, synthetic drugs, and natural products. Compatible with agarose, dextran, and polymer-based media, these columns are constructed from high-precision borosilicate glass and PEEK components, offering excellent biocompatibility and chemical resistance for most aqueous solutions.
Pressure ratings: WK-EC 6.6 – 35 bar; WK-EC 10 – 30 bar.
WK-EC 16/26/50 Series
WK-EC 16, 26, and 50 Series are low-pressure columns designed for process development in both biomacromolecule (recombinant proteins, antibodies, vaccines, blood products, etc.) and small molecule (antibiotics, peptides, synthetic drugs, natural products, etc.) applications. They are particularly suitable for natural polysaccharide-based media such as agarose and dextran. All columns in this series have a pressure rating of 5 bar.
Brand: WA
Separation Mode: Affinity
Base material: Agarose
Bead Structure: HP
Functional Group: Heparin
Specification: 1L
Cross Reference: Cytiva Heparin Sepharose High Performance, 1L
Gel Filtration Chromatography
Principles of GFC
Gel filtration chromatography (GFC) separates biomolecules based on differences in size and shape. The media consists of porous beads. Large molecules cannot enter the internal pores and elute quickly with the void volume, resulting in a shorter retention time. Smaller molecules penetrate the pores and elute later, as they spend more time within the matrix. Effective separation depends on choosing media with suitable pore sizes according to the molecular weight of the target substances.
Choosing Gel Filtration Resin-Based on Separation Purpose
- Group Separation
This involves classifying sample components into high and low molecular weight groups. It is commonly used for desalting and buffer exchange. Example: WD-G25 series. - Fine Separation
This aims to separate components with similar molecular weights and is used in analytical or fine purification stages. Example: WA-FF and WA-GF series.
Separation Range of Gel Filtration Media (for Globular Proteins)

Ion Exchange Chromatography
Principles of IEC
Ion exchange chromatography (IEC) separates biomolecules based on differences in their isoelectric points. At a given pH, different biomolecules carry different types and amounts of charges. This technique utilizes these differences for separation. Depending on the type of functional groups, ion exchange chromatography is categorized into cation and anion exchange chromatography.
Key Features:
- High controllability
- Strong selectivity
- High recovery rate
- High loading capacity
- Sample concentration capability
Ligands for IEC
| Ligand | Group | Category |
|---|---|---|
| S | Sulfomethyl | Strong cation exchange |
| SP | Sulfopropyl | Strong cation exchange |
| CM | Carboxymethyl | Weak cation exchange |
| Q | Quaternary ammonium | Strong anion exchange |
| DEAE | Diethylaminoethyl | Weak anion exchange |
Hydrophobic Interaction Chromatography
Principles of HIC
Hydrophobic interaction chromatography (HIC) separates substances by leveraging interactions between hydrophobic groups on biomolecules and hydrophobic ligands on the stationary phase. Salt ions disrupt the hydration shell around biomolecules, promoting interaction with hydrophobic ligands. Common ligands include butyl thioether, butyl, octyl, and phenyl groups.
Advantages
- Sample loading under high salt, elution under low salt—well-compatible with ion exchange chromatography
- Strong selectivity
- Excellent physicochemical stability
Factors Influencing HIC Performance
1. Salt Type and Concentration
Salts increase interfacial tension between proteins and resins, disrupting hydration layers and enhancing hydrophobic interactions, thereby promoting protein binding to the matrix.
Salts exert varying effects on HIC. Sulfates like Na₂SO₄, K₂SO₄, and (NH₄)₂SO₄ enhance hydrophobicity by strengthening protein salting-out effects and surface tension, whereas MgCl₂, while also strengthening surface tension, increases solubility and hence diminishes hydrophobicity and hinders protein binding.
High salt concentrations enhance hydrophobic interactions. A salt concentration near but below the target protein’s salting-out point optimizes binding without causing precipitation. Typically, a 1 mol/L ammonium sulfate solution is sufficient to promote binding of highly soluble proteins to resins.
2. pH
HIC typically uses neutral phosphate buffers. While pH changes have limited effect, higher pH may slightly reduce protein hydrophobicity. pH adjustments are rarely used for modulating HIC.
3. Temperature
Higher temperatures enhance hydrophobic interactions but may compromise protein activity. HIC should be performed under stable temperature conditions to avoid unwanted influences.
Affinity Chromatography
Principles of Affinity Chromatography
Affinity chromatography exploits the specific and reversible interactions between biological molecules-such as antigen-antibody, enzyme-substrate, hormone-receptor, or complementary DNA strands-to achieve high-purity separations.
Advantages of Affinity Chromatography
- Fast and efficient
- High selectivity
- Achieves >90% purity in a single purification step
Resin Structures
- Matrix
A solid support for ligand immobilization, typically agarose or dextran gels - Spacer Arm
A linker of appropriate length connecting the matrix to the ligand - Ligand
A molecule capable of specifically and reversibly binding the target biomolecule
Empty Columns
WK-EC 6.6/10 Series
WK-EC 6.6 Series and 10 Series are lab-scale empty columns specifically designed for medium- and low-pressure chromatography. They are applicable to biomolecules such as recombinant proteins, antibodies, vaccines, and blood products, as well as small molecules like antibiotics, peptides, synthetic drugs, and natural products. Compatible with agarose, dextran, and polymer-based media, these columns are constructed from high-precision borosilicate glass and PEEK components, offering excellent biocompatibility and chemical resistance for most aqueous solutions.
Pressure ratings: WK-EC 6.6 – 35 bar; WK-EC 10 – 30 bar.
WK-EC 16/26/50 Series
WK-EC 16, 26, and 50 Series are low-pressure columns designed for process development in both biomacromolecule (recombinant proteins, antibodies, vaccines, blood products, etc.) and small molecule (antibiotics, peptides, synthetic drugs, natural products, etc.) applications. They are particularly suitable for natural polysaccharide-based media such as agarose and dextran. All columns in this series have a pressure rating of 5 bar.
Brand: WA
Separation Mode: Affinity
Base material: Agarose
Bead Structure: HP
Functional Group: Heparin
Specification: 1L
Cross Reference: Cytiva Heparin Sepharose High Performance, 1L

